News and events
Interested in knowing more about our breakthroughs in research or where to meet us at events? Sign up to our newsletter below or discover more right here.
-
News
Cercare Medical at AOCR 2024: Unveiling the Future of Radiology with Our Experts
Meet Cercare Medical at AOCR 2024
News archive
-
Cercare Medical at AOCR 2024: Unveiling the Future of Radiology with Our ExpertsLearn more
-
Meet our French Team at SFNR 2024Learn more
-
Engage With Cercare Medical at ECR 2024Learn more
-
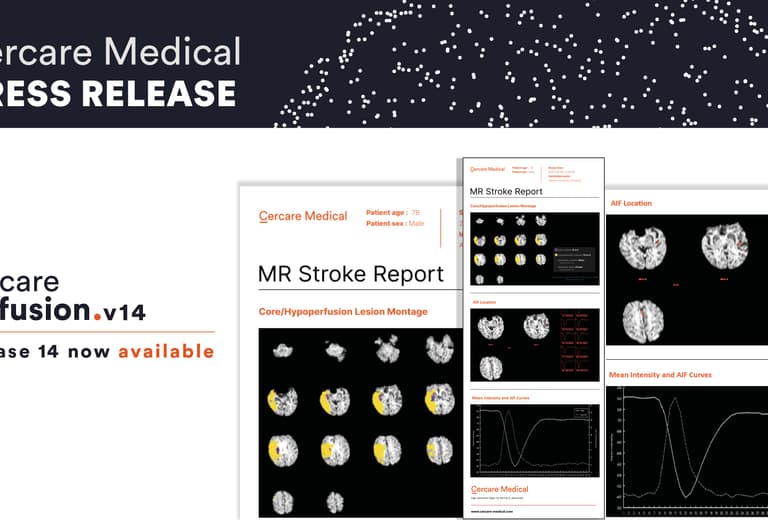
Cercare Medical News: Revolutionizing Stroke Care: Cercare Medical Debuts CMN Version 14Learn more
-
Cercare Medical News: Celebrating Innovative Collaboration with Fondation Rothschild HospitalLearn more
-
Meet us @Billeddiagnostisk Årsmøde 2024Learn more
-
Meet us @Munchen Radiologie SymposiumLearn more
-
Meet us @Sädeturvapäivät Congress 2023Learn more
-
Meet us @JFRLearn more
-
Meet us @NeuroradLearn more
-
Meet us @World Stroke CongressLearn more
-
Cercare Medical @ESMINTLearn more
-
“Cerebrovascular Reserve in Moyamoya Disease: Relation to Cerebral Blood Flow, Capillary Dysfunction, Oxygenation, and Energy Metabolism."Learn more
-
Cercare Medical @DGNCLearn more
-
Cercare Medical @NCRLearn more
-
Cercare Medical @ESOCLearn more
-
Cercare Medical @ASNRLearn more
-
Cercare Medical @DMEALearn more
-
SFNR 2023 with Cercare Medical!Learn more
-
Meet us @ECR 2023!Learn more
-
Cercare Medical @ArabHealth 2023!Learn more
-
RSNA 2022: New global partnership agreement with Viz.ai!Learn more
-
Cercare Perfusion Featured on TV4’s Morning NewsLearn more
-
Cercare Medical Partners with Karolinska University Hospital for Clinical Perfusion ImagingLearn more
-
Cercare Medical Partners with Arterys to Provide Its Innovative Perfusion Imaging Solution on Arterys’ Cloud-Based PlatformLearn more
-
Massachusetts General Hospital to Use CERCARE Perfusion in Neuroradiology ResearchLearn more
-
Cercare Medical Receives FDA Clearance for Its AI-Powered Perfusion Imaging SolutionLearn more
-
Cercare Perfusion Implemented at Aarhus University Hospital for a Pilot ProjectLearn more
-
Cercare Medical Partners with BlackfordLearn more
-
Cercare Medical Partners with Wellbeing SoftwareLearn more
-
Cercare Perfusion is Now Available on syngo.via OpenAppsLearn more
-
Meet us at RSNA 2019Learn more
-
Cercare Medical Receives a €1,8 Million EU GrantLearn more
-
Philips Healthworks Breakthrough Day - VideoLearn more
-
Cercare Medical transforms the future of healthcare with MedtronicLearn more
-
Cercare Medical is Partnering with Siemens HealthineersLearn more
Want to stay informed?
Sign up for our monthly newsletter, Cercare Bulletin, to stay updated with the latest news in the neuroradiology field and AI.